- Information
- AI Chat
Engineering Materials - must haves
Engineering Studies
Recommended for you
Preview text
ENGINEERING STUDIES - MATERIALS
general MATERIAL info METALS -! electrical & thermal conductivities - Ductile - Stiffness, toughness & strength Engineering metals: alloys - metals are too weak in pure state. E. pure iron = weak & soft, +dding carbon = tough steel. Ferrous Alloys: >50% iron: - Plain carbon steels - Alloy steels - Cast irons Non-Ferrous Alloys: <50% iron: - Light alloys of Al, Mg, Ti and Zn - Heavy alloys of Cu, Pb, Ni ($$$) - Heat resistant metals - molybdenum, tungsten - Precious metals - gold, silver, platinum POLYMERS & ELASTOMERS - Organic materials that are !"#$%&'&(%")"%(&+,-.("/+$/(+0&("1+2--(+*"3"(.,0&4 - Thermosoftening plastics: soften w/ heat (flexible + rel. soft) - Thermosetting plastics: do NOT soften w/ heat (rigid + hard) Elastomers: structure allows large extensions that are reversible (RUBBER BANDS!!)
Polymers:"6(.(40+&4,."7"08(+#,."4$9%240&:&0;"<"softer, less dense + !"corrosion resistance than metals. CERAMICS - HARD + BRITTLE - !4$#/+(&:("3"60(9*&.("0+(9=08 - 6.$>"(.(40+&4,."3"08(+#,."4$9%240&:&0; - Chemically inert - do not react w chemicals E. glasses, cements, fired clays (pottery), electronic ceramics (semiconductors, superconductors), engineering ceramics. COMPOSITES - Bonding 2+ materials to create a final material = combo of good properties of bonded materials E. glass reinforced polymer (GRP), reinforced concrete, asphalt, wood. Generally = !*/(4&'&4"0+(9=08"10+(9=08?#,
CIVIL STRUCTURES
Testing of materials - specialised testing, x-ray & testing concrete:
Crack Formation & Growth: - Method of brittle failure. - When crack forms, strain energy is retained but released from the area adj to the crack Failure due to Cracking: - More brittle = shorter critical crack length - Critical crack length exceeded = failure inevitable if stress levels maintained Repair/Elimination of Failure due to Cracking: Repair Metal: welding. - Weld repairs crack but changes the microstructure = weaker material. Might need heat treatment to solve this issue Polymer: adhesives. - If failure in thermoset + no adh available - replacement of material - Thermoplastic material - polymer welding = strength close to parent material Ceramic: glue/cement - Repairs in stone + concrete = difficult. - Replacement!! Composite: replacement. - Only efficient way Elimination Design w/o sharp corners: corners concentrate stress Placing an interface for material: an area within a material, weaker than surrounding area that runs perpendicular to growth of crack. - When crack travels through material = blocked and never reach critical crack length
Ceramics - structure/prop, glass, cement, bricks: Structure/properties: - Hard, brittle, chemically inert, electrical & thermal INSULATION, durable - Compressive strength GLASS
- Non-crystalline ceramics
- 3 basic ingredients: silica, limestone, soda ash
- Soda-lime glass: acc for 90% glass - windows, bottles etc.
- Borosilicate glass: used for ovenware, telescopes
- Lead glasses: optical comp, radiation shielding
- Main properties: transparent, brittle, compressive strength
- Properties can be improved: thermal toughening, chemical toughening, laminating CEMENT
- Bonding material
- Compressive strength
- Low toughness
- Easily casted
- Excellent workability Composites - timber, concrete, asphalt, laminates, geotextiles: TIMBER - Organic material - Structure: cellulose tubes bounded together by glue lignin (wood grain) - Factors affecting strength: loading duration, moisture content, defects within grain - Exposure to chemicals
Corrosion - corrosive env, dry, wet, stress corrosion: Corrosive environments: - Availability of oxygen to enable reactions to proceed - Temperature - Oxidation occurs when the metal loses electrons, and occurs at ANODE - Reduction is the consumption of electrons, and occurs at CATHODE OIL RIG = oxidation is loose, reduction is gain Dry, wet and stress corrosion: Dry Corrosion Wet Corrosion Stress Corrosion Occurs through chemical reactions with gases @ high temps E. furnaces Occurs when material is in contact with fluid/moisture using an electrolyte Uniform attack - when metal placed within electrolyte and some parts anodic/cathodic Galvanic corrosion - when dissimilar metals placed in presence of corrosive env When material subjected to stress & cracks begin to form. Material eventually degrades due to fatigue Protecting civil structures - painting the surface of the material OR galvanising - dipping metal into molten zinc that covers the steel and protects it from corrosion by acting as a passive layer. Recyclability of materials: Steel: - BOF (basic oxygen furnace) - 25% recycled steel - EAF (electric arc furnace) - 100% recycled steel Concrete:
- Recycled conc weaker than OG product
- Usually used as rubble
- Conc = crushed/broken down & re-used Wood:
- Can be recycled for basic uses - furniture, pallets
- Dependent on type of wood
- Chips for garden mulch, playground covering
- Smaller chips to form wood composites
- Recycled as paper/cardboard Asphalt:
- Limited use for recycled products
- Crushed and refined w other materials added to reproduce asphalt Glass:
- Reused to produce glass again
Visual testing ● Dye penetration ○ Dye or coloured liquid is placed on the surface of a component and excess is wiped clean ○ Any cracks or imperfections on surface of component will be highlighted by the dye remaining ○ Fast, simple, inexpensive ○ Used for small specimens and various materials ○ Difficult to detect small cracks ○ UV light is also used to help show up any imperfections ● Magnetic particle testing ○ Component is placed on a conducting rod, that produces a magnetic field about the component ○ Fluorescent liquid of charged particles is sprayed over component ○ Fluorescent magnetic particles are drawn to the cracks by the conducting rod, highlighting surface imperfections Radiographic examination ● X-Rays ○ Favourable because a photo film is produced, for close analysis ○ Detects subsurface defects ○ Radiation is used to penetrate the item, with any voids allowing the rays to pass through more easily, resulting in a dark area of film ○ Used on large objects
○ Longer/more expensive than visual testing ○ Exposure to radiation can be harmful to humans ● Gamma Rays ○ Effective when testing thick structures, i., steels ○ Can be used to examine joining methods, i., welds ○ Exposure to radiation can be harmful to humans Ultrasonic testing ● Detects subsurface defects ● A probe transmits high frequency vibrations throughout the component as it passes over the surface of a component ● Any imperfections within the component causes the vibration to be reflected without travelling to the bottom ● Results are displayed on detection machine Heat treatment of ferrous metals - annealing, normalising, hardening & tempering, changes in macro/microstructure, changes in properties: ANNEALING Annealing = heating & cooling of a metal to produce SOFTEST STATE. (i) relief of int stresses (ii) produce UNIFORM GRAIN structure (iii) to soften material 4 further working/machining Process Annealing: BELOW RED HEAT
Heat steel btwn 550-650 degrees C
Relieve INTERNAL stresses in material
Air cooled
Complete recrystallisation of material
Smaller grains than Pannealing = harder & stronger steel – finer grain structure
Refine grain structure (uniform + equal in size), improve machinability
Increase in UTS
Decrease in ductility HARDENING/TEMPERING Hardening:
Heating steel w sufficient carbon to red heat (austenising) and cooling quickly (quenching)
Extremely hard & brittle material = rapid cooling = martensite – needs further treatment to become tougher Tempering:
Heating hardened steel to temperature below 723 degrees C, soaking to remove INT stresses + to allow structural changes to go to equilibrium followed by cooling. !0(#/(+&9="8(,0")" 6 0(9*&.("*0+(9=08B";&(.%" *0+(**" 7 "8,+%9(**B"!%240&.&0;C
Annealed → coarse grain structure → soft with moderate strength
Normalised → fine grain structure → higher strength
Hardening → stressed grain structure → hardness + brittleness
Tempering → very fine grain structure → toughness + hardness
Manufacturing process for ferrous metals – forging, rolling, casting, extrusion, powder forming, welding: FORGING Forging: - Forming metals by compressive forces = above recrystallisation temp - Produces grain flow in metal = better mechanical prop than produced by casting/machining. = valves, bolts, gears, connecting rods. Closed Die Forging:
Squeezing of hot metal between 2 shaped dies and the excess metal trimmed off in trimming die Drop Forging:
Form of closed die forging
Upper die dropped onto lower die Open Die Forging:
When metal hammered/pressed by a vertically moving tool onto a stationary tool ROLLING Rolling: - Metals pressed into shape through rollers. LIKE PASTA/DOUGH!! Hot Rolling:
no directional prop - POOR surface finish
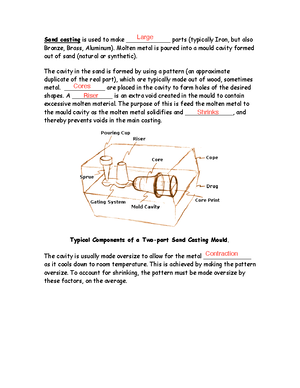
- defects: cracking, porosity, blow holes, piping. Shell Moulding:
- Making a copy of desired part and surrounding it with special sand that has thermosetting resin that hardens when heated. Investment casting:
- Special wax runs through accurate metal mould to produce exact WAX replica. Die Casting:
- Metal forced into mould cavity under pressure Centrifugal Casting:
- Molten metal injected into SPINNING mould
- Forces molten metal to stick to interior of metal
EXTRUSION Extrusion: metal forced through die, so it takes shape of the die as it passes POWDER FORMING Powder Forming: - Metal powder mixed with other materials and poured into mould at RT - Mixture pressed into mould 4 desired shape - Pressure = particles together - Pressed item = sintered in controlled atm furnace - Heated 2 temp. Where atoms are allowed 2 diffuse between grains, producing uniform grain structure - Used = form brake pads → materials with different properties mixed together to give superior final product - Difficult to produce certain shapes WELDING Electric Arc Welding: - High elec current produces an arc btwn work & electrode that jumps from electrode 2 work. - ↑ temps that melt the electrode and part of the work metal = VERY strong joint
- ↑ temp flame to melt parent metal w filler rod being used to provide extra metal 4 joint Solid State Welding:
- Bonding 2 metals in SOLID STATE Pressure welding – ductile metal pressed onto similar/dissimilar metal – cladding of Al drink cans Friction Welding – 2 spinning surfaces/spinning tool over surfaces to be joined = sufficient heat to form bond as strong as parent metals Explosive Welding – 2 surfaces forced together by controlled explosive charge. Manufacturing processes for non-ferrous metals: alloying, annealing, solid solution hardening: Aluminium Brass Bronze
- Non-corrosive
- Lightweight
- Good strength to weight ratio
- Easily fabricated
- Very good electrical conductivity
- Ductile
- Alloy of copper and zinc
- Corrosion resistance
- Cannot spark
- Low coefficient of friction
- Alloy of copper and tin
- Excellent corrosion resistance→ from oxidization
- Hard
- Brittle
Aluminium silicon: - Good casting properties - More corrosive than pure aluminium Aluminium copper: - High strength - Good electrical conductivity - More corrosive than pure aluminium - Hard Aluminium silicon- magnesium: - Medium strength - Weldable - Car doors Annealing: Annealing is used to relieve any internal stress in a cold worked alloy. This results in an equiaxed grain structure. Precipitation hardening:
- Step 1-Solution Treatment: The alloy is heated to 530 degrees until the β phase dissolves to produce a homogenous sing phase alloy. It’s then quenched to room temperature.
- Step 2-Aging: Over time the trapped β phase precipitates out on stress planes within the quenched phase, thus restricting dislocations and strengthening the alloy
Engineering Materials - must haves
Subject: Engineering Studies

- Discover more from: