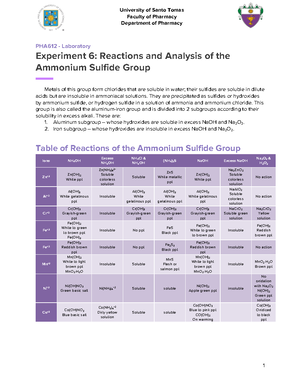
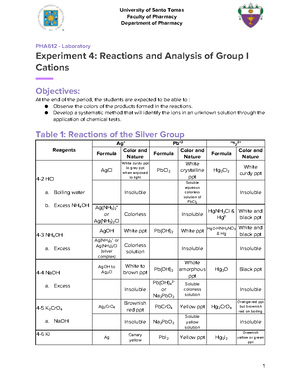
- Information
- AI Chat
Was this document helpful?
AAshortcuts - Shortcuts / summary of amino acids
Course: BS Nursing (BSN)
462 Documents
Students shared 462 documents in this course
Was this document helpful?

A list of tricks to help you remember the amino acids
Structures
Names (letter code)
Side chain features/description
Aliphatic
C
H
H
COOH
NH2
Glycine (G)
hydrogen for R, most simple, optically inactive
C
H
CH3
NH2
COOH
Alanine (A)
methyl
for R, a simple functional group to start just
like “A” (in alanine) starts the alphabet
C
H
NH2
CH
CH3
CH3
COOH
Valine (V)
simple again, but shaped like the “V” in its name
C
H
NH2
COOH
CH2
CH
CH3
CH3
Leucine (L)
valine extended by one
methylene
C
H
NH2
COOH
CH
CH3
CH2
CH3
Isoleucine (I)
lopsided
valine....?
N
COOH
H
Proline (P)
3 carbon chain to proline’s own nitrogen,
structurally “special” and found in turns
Sulfur-containing
C
H
NH2
COOH
CH2
CH2
S
CH3
Methionine (M)
special - starts every protein, 3 carbons with a
thioether;
methyl-blocked
sulfhydryl...?
C
H
NH2
COOH
CH2
HS
Cysteine (C) “
sulfhydryl
alanine,” reactive, can form disulfides
© 2001 by Jeff Dahlseid