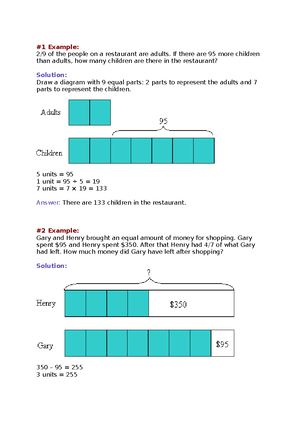
- Information
- AI Chat
Specialized GEN CHEM 2
BSE Science
University of Caloocan City
Preview text
Fig. 2 Water the Most Miraculous Molecule.
GENERAL CHEMISTRY 2
MODULE 2: INTERMOLECULAR FORCES IN
LIQUIDS AND SOLIDS
In a 100 year period, a water molecule spends 98 years in the ocean, 20
months as ice, about two weeks in lakes and rivers, and less than a week in
the atmosphere. (Only 1% of the earth’s water is usable).The earth is a closed
system (rarely loses or gains matter). The same water that existed on the earth
millions of years ago is still present today. (www. Science facts. Com)
INTRODUCTION
Water because of its peculiar properties is considered to be the most miraculous molecule and abundant substance in the universe. It comprises 70% of the Earth’s surface and makes up 75% of our bodies. The intermolecular forces of attraction between its molecules enable it to
a. dissolve more materials than any other solvent, b. create tension in its surface, c. flow easily, d. rise in a tube, e. exert pressure, f. escape in the atmosphere, g. boil at a specific point, h. provides insulation and warmth of the Earth’s surface, i. and maintain balance in the universe.
Learning Targets: The main goal of this module is to meet the following competencies:
- Describe the following properties of liquids and explain the effect of intermolecular forces on these properties: surface tension, viscosity, vapour pressure, boiling point, and molar heat of vaporization (STEM_GC11IMFIIIa – c – 102)
- Explain the properties of water with its molecular structure and intermolecular forces (STEM_GC11IMFIIIa – c – 103) 3 the difference in structure of crystalline and amorphous solids (STEM_GC11IMFIIIa – c – 104) To achieve these competencies the following objectives must be met:
identify the properties of liquids and solids;
relate intermolecular forces to properties of liquids and solids;
cite daily life application of these properties;
identify the physical and chemical properties of water and
explain the properties of water based on intermolecular force.
Try This
MULTIPLE CHOICE: Choose the letter of the correct answer. Write your answer on your notebook.
- Water drops stay together on wax paper and they don’t break apart easily. This is mainly because: a) Water molecules are small b) Water molecules are in motion c) Water molecules are attracted to each other d) Water molecules are wet
- When you bring two drops of water near each other and allow them to touch, they combine immediately and become one drop. This is mainly because: a) Water molecules are made of atoms b) Water molecules are attracted to each other c) Water molecules are magnetic d) Water is a liquid
- If you put food coloring in room temperature water, the coloring spreads throughout the water. The water causes the color to spread mainly because: a) Water molecules are warm b) Water molecules are in motion c) Water is more dense than food coloring d) Food coloring molecules are small
- Food coloring spreads out faster in hot water than in cold water. This is mainly because: a) The water molecules in hot water move more quickly b) The molecules in hot water are larger c) The food coloring molecules are small d) Hot water is less dense
- When a thermometer is heated, the red liquid inside the thermometer moves up. This is mainly because: a) The red liquid is thin. b) The molecules of the liquid move faster and get a little further apart c) Hot liquid is lighter d) The glass of the thermometer gets hot
- When a thermometer is cooled, the red liquid inside the thermometer moves down. This is mainly because: a) Cold liquids sink b) The glass of the thermometer gets cold c) The molecules of the liquid move slower and get a little closer together d) The red liquid is thick
- When you heat a sample of a solid, the particles that make up the solid:
Fig. 2 Surface Tension
Discussion
The remarkable properties of liquids particularly water can be attributed to the
intermolecular force presents on it. This force, the H- bond as we have
discussed is the strongest of the four types of intermolecular forces. The
properties of liquids include the following:
- Surface Tension – Seen in Fig. 2, when water is placed on waxy surface, it “beads up” forming distorted sphere. This behaviour is caused by the unbalanced intermolecular forces at the surface of the liquid. Molecules in the interior are attracted equally in all
directions, as shown in Fig. 2 whereas those at the surface experience a net inward force. This inward force pulls molecules from the surface into the interior reducing the surface area. The inward force also make the molecules at the surface packed closely together, causing the liquid to behave almost as if it had a skin. This effects permits some insects to “walk” on water even though their densities are greater than that of water Tension depends on the temperature of the surroundings as temperature increases surface tension decreases. 2. Capillary Action – Intermolecular forces that bind similar molecules to one another, such as the hydrogen bonding bonding in water are called cohesive forces, while forces that bind a substance to a surface are called adhesive forces placed in a glass tube adheres to the glass because the adhesive forces between the water and glass are even greater than the cohesive forces between water molecule. This difference on the attraction between molecules of the liquid and between molecules of liquid and glass give rise to capillary action. This explains why water with dissolved minerals can pass through xylem and phloem of plants. In this way plants can get the nutrients it needs. 3. Diffusibility – Liquid molecules slide over one another causing them to diffuse easily. The diffusibility of liquids is lower than gases but diffusion helps molecules of liquid to dissolve easily in another liquid or solid in a liquid. 4. Incompressibility – liquids are incompressible because molecules are held together by a strong intermolecular force. 5. Evaporation – as the temperature of the liquid rises surface tension decreases and the molecules gain kinetic energy, thus it moves rapidly. The rapid movement of molecules overcomes the intermolecular force and surface tension in liquid this makes them escape from the liquid and
undergo phase changes. Those molecules who escaped from the liquid will exist as vapour. As evaporation proceeds, the kinetic energy and temperature of molecules left will be lower this is the reason why evaporation has a cooling effect. 6. Boiling Point – is the temperature at which the vapour pressure of the liquid equals the atmospheric pressure. The boiling point of the liquid decreases as the altitude increases because atmospheric pressure decreases, this is why boiling an egg at higher altitude takes a little longer and it will boil at less than 100 ̊C (boiling point of water at sea level). 7. Viscosity – This is the resistance of liquid to flow. There are liquids such as honey and motor oil flow very slowly, others such as gasoline and water flow very easily.
Table 2:1 : Table Completion: Complete the table below. Reproduce the
table and write your answer on your notebook.
Properties Characteristics Application
Fig. 2 Water molecule and hydrogen bonds shown by the dashed lines
PHYSICAL PROPERTIES
Water is a colorless and tasteless liquid. The high hydrogen bonds of its molecules result to unusual properties in the condensed form. This leads to high boiling point and melting point. It has high in a specific heat, thermal conductivity, surface tension and dipole moment. These properties make water the most important compound in the living world. Being an excellent solvent it dissolves important ions and compounds in the body required for proper metabolism. Its high heat of vaporization helps the body to regulate its temperature.
Table 2. Physical Properties of Water
Property Value Temperature (∞C)
Effect and Significance
Density 0 0 Solid form is less dense than its liquid form; ice floats at the surface of liquid water. Water expands upon freezing; ice in temperate countries floats on rivers, oceans and streams during winter months preventing its complete solidification. Thus, aquatic or marine life is preserved.
1 g/mL 3. 0 g/mL 25 0 100
Viscosity I. 79 cP 0 Hot molasses flows much faster than 0 cP 25 cold molasses. 0 cP 100 Surface tension
75 dyn/cm 0 Water bugs (striders) seem to skitter across this skin as if ice-skating. You can actually float a pin on water, if you carefully lay it across the surface. This is the reason why rains form drops.
72 dyn/cm 25 58 dyn/cm 100
Dipole moment
1 D Reflects the polarity of water molecule. This makes water a universal solvent. Specific heat 1 cal/g/∞C Water can absorb a substantial amount of heat while its temperature rises only slightly. This maintains balance in the atmosphere. This also provides the insulation and warmth of the Earth. Latent heat of fusion
80 cal 0
Latent heat 540 cal 100
of vaporization Boiling point 100∞C at 1 atm
CHEMICAL PROPERTIES
Water reacts with a lot of substances to form different compounds. Some
significant reactions are as follows:
Amphoteric nature:
Water can act as both acid and base, which means that it is amphoteric
innature.
Example:
Acidic Behavior: H 2 O(l)+NH3(aq) ⇌ H 3 O+(aq)+NH+4(aq)
Basic Behavior: H 2 O(l)+H 2 S(aq) ⇌ H 3 O+(aq)+HS−(aq)
Redox reactions:
Electropositive elements reduce water to hydrogen molecule. Thus water is a
great source of hydrogen. Let us see an example in this case:
2H 2 O(l)+2Na(s)→2NaOH(aq)+H2(g)
During the process of photosynthesis, water is oxidized to O 2. As water can be
oxidized and reduced, it is very useful in redox reactions.
Hydrolysis reaction
Water has a very strong hydrating tendency due to its dielectric constant. It
dissolves many ionic compounds. Some covalent and ionic compounds can be
hydrolyzed in water.
Reacts with non – metal to form hydroxides
A soluble substance such as CaO and Na2O reacts with water to form
hydroxides.
Reacts with non – metallic oxides to form acids
Non – metallic oxides such as CO 2 , SO 2 , and N 2 O 5 react with water to
form acids.
Fig. 3 Unit cell formation of crystalline solid
Table 3 Types of Crystalline Solid
Type of solid Form of unit particles
Forces between particles
Properties Examples
Molecular Atoms or molecules
London, dipole- dipole, hydrogen bond
Soft, low to moderately high melting point, poor thermal and electrical conduction
Argon, methane, sucrose and dry ice
Covalent network
Atoms connected in a network of covalent bonds
Covalent bonds
Very hard, very high melting point, often poor thermal and electrical conduction
Diamond and quartz
Ionic Positive and negative ions
Electrostatics attraction
Hard, brittle, high melting point, poor thermal and electrical conduction
Table salts
Metallic Atoms Metallic bonds Soft to very hard, low to very high melting point, excellent thermal and electrical conduction, malleable and ductile
All metallic elements
AMORPHOUS SOLID
Solid can also be amorphous (from the Greek words meaning “without
form”) where particles are randomly arranged. These solids lack well – defined
faces and shapes. Many of amorphous solids are mixtures of molecules that do
not stack together well. Most others are composed of large, complicated
molecules. Examples of amorphous solids are rubber, glass and plastics.
Because the particles of amorphous solid lack any long – range order,
intermolecular forces vary in strength throughout a sample. Thus amorphous
solid do not melt at specific temperatures. Instead, they soften over a
temperature range as intermolecular forces of various strengths are overcome.
Apply what you have Learned Explain the following 1. Raindrops that collect on a waxed automobile hood take on a nearly spherical shape. 2. The capacity of paper towels to absorbs water. 3. How intermolecular force affects the vapour pressure of the liquid. 4. Why some solids are dissolve in liquids others do not?
Assess What You Have Learned
I. MULTIPLE CHOICE: Choose the letter of the correct answer. Write your answer on your notebook
- How do insects such as pond skaters stay afloat on water? a. Because of high surface tension of water b. As they can swim c. Because they are less dense than water d. None of the above reasons
- This image specifically demonstrates one example of how ________________ is beneficial to living organisms. a. Adhesion b. Surface tension c. Specific heat d. Capillary action
- The first falling leaf of the season has tumbled from the air and landed on the top of the pond. It does not sink, but yet float on top of the surface. What is happening on a molecular level that is preventing the leaf from sinking? a. The covalent bonds between the oxygen and hydrogen atoms are not breaking apart due to the lack of weight placed upon them. b. Both oxygen and hydrogen are positively charged and therefore much stronger c. There is not enough weight to break the hydrogen bonds between water molecules at the surface of the water
a. molecules with higher kinetic energy leaves the bod b. molecules with lower kinetic energy left in the body c. molecules with higher kinetic energy left in the body d. molecules with lower kinetic energy leaves the body 15. As you go to higher elevations above sea level the boiling point of water a. decreases. b. increases. c. stays the same. d. changes with the initial temperature of the water. 16. Increasing the rate of heating under a pot of boiling water will a. increase the temperature of the boiling water. b. increase the rate of boiling, but not the temperature. c. increase both the rate of boiling and the temperature of the boiling water. d. all of the above. 17. Which of the following is the condition for the boiling of a liquid? a. Absolute pressure of a liquid must be greater than or equal to it’s vapor pressure b. Absolute pressure of a liquid must be less than or equal to it’s vapor pressure c. Absolute pressure of a liquid must be equal to it’s vapor pressure d. Absolute pressure of a liquid must be greater than it’s vapor pressure 18. What factor affect the high boiling point of water? a. the strong dipole bond in water molecules b. the dispersion bond between water molecules c. the ions present in it d. the molecules present in it 19. What is responsible for the difference in viscosity of liquids? a. kinetic energy b. temperature c. intermolecular force d. molecules 20. On a large scale, the Earth is 75% water. What property of water provides the insulation and warmth of the Earth’s surface? a. specific heat c. surface tension b. cohesion d. adhesion 21. Solids have many different properties, __solids are known for their ability to be flattened into a sheet, stretched into a wire, and to conduct energy well. a. a. Molecular c. metallic b. b. network d. ionic 22 of the following is true about solids? a. Solids maintain a defined shape and size under all conditions. b. All solids have a crystalline structure. c. All solids maintain a defined shape and size if conditions remain constant. d. All solids have a lattice structure at atomic level.
23 property of molecular solids is not true? a. intermolecular forces are weak b. they have low melting point c. they do not conduct electricity d. they are made up of many different types of 24 of the following is not a characteristic of crystalline solids? a. particles are arranged in repeating pattern b. has distinct melting point c. made up of crystals d. becomes softer and softer as temperature rises 25 solids conducts electricity when a. present in pure form. c. dissolves in water. b. it is in crystal form. d. it forms an alloy. 26. Which of the following is a crystalline solid? a. Copper wire c. Glass bottle b. Polythene bag d. Rubber ball 27. Metals can be hammered into different shapes and drawn into wires hence they are a. Ductile c. Luster b. Malleable d. Conductor 28. The following are the characteristics of metallic solids except: a. Malleable c. Conductivity b. Luster d. Soluble 29. What happens to the molecules of molecular solids when molten? a. Move- apart from each other. b. Broken up. c. Move – closer to each other. d. None of the above option 30. The hardest solid known to man is a. Graphite c. Sulfur dioxide b. Benzene d. Diamond II. Table Completion: Reproduce the table on your notebook and complete
the table below
Types of Crystalline Solid
Bond Between Particles
Description Examples
ABOUT THE AUTHOR
Maricel P. Santillan. A graduate of Bulacan State University with the degree Bachelor of Science in Education major in Physics. She is a recipient of Japan- Philippines Exchange Student Scholarship Program in Teaching Physics(2002). She is a Senior High School Teacher III of Meycauayan National High School, handling Accounting 1, Business Finance, Physical Science, Earth and Life Science, Entrepreneurship and General Chemistry 2 subjects in Grade 11 and 12 since
- She is a Basic Education Research Fund Grantee in 2017. She is a
writer / author of Physics, Chemistry and Mathematics books under St.
Andrew Publishing Company. She was awarded outstanding in performance
(RPMS) school year 2018 – 2019. She is now in her Thesis Writing for her
MAED – Physics at the Bulacan State University.
CONTENT EDITOR Mrs. Reychelle A. Serrano is a Master Teacher I in Meycauayan National High School – Senior High School Department. She is currently on her 10th year of teaching after finishing her Bachelor of Secondary Education Major in Physical Sciences at Bulacan State University. She also graduated Master of Arts in Education major in Physics in the same academe. She is presently handling science subjects like Earth & Life Science, Earth Science, Physical Science, General Chemistry 1, General Biology 1 and General Physics 1 and 2 and the Subject Group Head of all SHS Science teachers. She is a consistent Division Science Quiz Bee coach winner since 2011 for Grades 10, 11 and 12, and the trainer of “2019 National Battle of Science Quiz Bee Champions” winner who bagged 3 rd Place for the SDO - City of Meycauayan. Also, she is a Division Research Finalist in the 2019 Division Research Congress funded by Basic Education Research Fund (BERF) and a Division Resource Speaker. She also became a Division Strategic Intervention Material (SIM) author for Grade 12 STEM in SHS entitled “Project-ILE Motion”. Likewise, she is a Division Module Content Evaluator in Science for Elementary, Junior High School and Senior High School Self-Learning Modules for SDO- Meycauayan. In addition, she was also a consistent awardee as Outstanding Master Teacher based on RPMS since 2016.
Specialized GEN CHEM 2
Course: BSE Science
University: University of Caloocan City

- More from:BSE ScienceUniversity of Caloocan City350 Documents